With this post, we continue to examine the ESG risks and opportunities inherent in the worldwide race to develop a treatment or vaccine for COVID-19.
The pandemic has brought to light systemic risks related to healthcare and investors are likely to increase engagement on pricing and affordability, while also executing trading strategies.
In our first blog on this topic, we highlighted the Healthcare industry’s favorable performance relative to other industries in the stock market volatility of recent months, reflecting investor expectations that companies will be able to profit from diagnostics and a potential treatment or vaccine. But the industry has fared well in another metric as well, which might even prove more valuable in the long term— public opinion.
According to data from The Harris Poll, 40% of American consumers say their view of pharmaceutical industries became more positive since the outbreak of the pandemic. The industry’s reputation has suffered for years, especially in the U.S. where voters point to the high cost of prescription drugs. Prior to the outbreak, the pharma sector ranked 10th out of 12 sectors in public opinion, followed only by government and tobacco[i].
This reputational boost may be the golden egg pharma needed, but it also reflects society’s heightened expectations for a vaccine that is not only safe and effective, but accessible to all.
In our first blog, we argued that firms with attractive Product Governance scores may be better able to navigate the complex regulatory and stakeholder pressures that are bound to intensify as companies work towards launching a product.
Now, we highlight another Material ESG Issue (MEI)— Access to Basic Services, which will become increasingly important as companies advance in their clinical trials. This MEI captures the risk associated with failing to ensure patient access to essential treatments, in both developed and emerging markets.
In addition to safety and efficacy, pricing will be paramount in controlling the pandemic, and locking in pharma’s reputational gains
Pharma companies are facing enormous, sometimes conflicting, pressure from shareholders, investors, society and regulators. Institutional investors with over 2 trillion USD in assets recently urged the industry to follow principles of transparency and cooperation, rather than focusing on short term gain, in order to find a sustainable solution to the pandemic[ii].
No pricing or access decision has escaped close scrutiny, positive or negative, and industry seems to be paying attention. J&J committed to providing a vaccine at a no-profit price of $10. When Gilead first requested lucrative orphan drug status from the U.S. FDA for Remdesivir, which would have granted seven years of market exclusivity, it quickly rescinded the request due to significant public backlash.
ESG scores may help investors with security selection
Sustainalytics’ unmanaged risk scores for Access to Basic Services assess exposure to and management of risks related to pricing and affordability. We assess a company’s access strategy based on six indicators related to intellectual property access, R&D, drug price transparency, equitable pricing, and value-based programmes, with a discounting factor for involvement in controversies.
We believe that firms with attractive Access to Basic Services scores may be better equipped to implement a sustainable access strategy and face a lower risk of reputational, regulator, and investor scrutiny. At the same time, these scores should be considered holistically along with other key issues like Product Governance, and overall unmanaged ESG risk.
While there are over 200 treatments and 140 vaccines in development for COVID-19, less than a dozen are currently being tested on humans[iii]. The table below shows select firms that are in Phase II or III clinical trials. As companies come closer to launching a new product, a pricing and access strategy becomes more material.
Gilead’s Remdesivir in the only treatment that has been approved for emergency use so far, in the U.S. and Japan. Pfizer and Moderna are testing mRNA vaccines in Phase II trials. AstraZeneca has an agreement to commercialize a vaccine currently being tested at the University of Oxford.
Regeneron and Sanofi are collaborating on late-stage trials to repurpose Kevzara, an IL-6 inhibitor. Roche (Actemra), Amgen (Otezla), Alexion (Ultomiris) and Novartis (Canakinumab) are also testing existing drugs that could help treat critical COVID-19 patients.
However, as shown below, these firms face different levels of ESG risk. Sanofi, Novartis, and Roche are tops when it comes to Access to Basic Services. Despite moderate drug pricing controversies, these companies have experience implementing access programmes in developed and emerging markets.
Existing access strategies could be replicated, giving these companies a competitive advantage over less internationally oriented companies like Alexion and Moderna.
Select MEI and ESG Risk Rating scores in Pharmaceutical industry
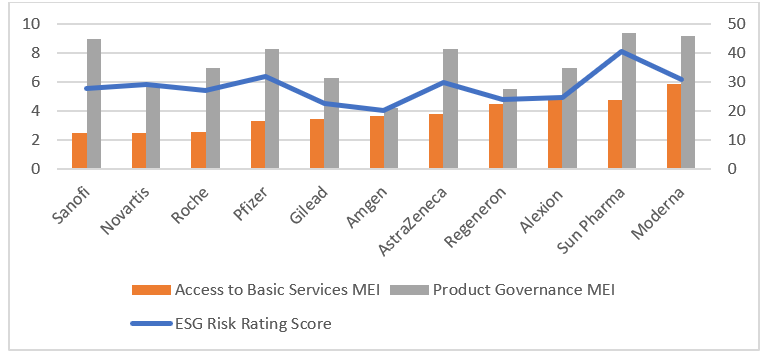
To summarize, we believe ESG issues will only become more important in a post COVID-19 world. Two MEIs in particular, Product Governance and Access to Basic Services, will be paramount in developing a treatment or vaccine that is both safe and accessible. Companies with a strong track record on these two issues may be relatively well-positioned to come out ahead.
Moreover, if firms are able to heed global calls to place cooperation and shared innovation above short term gains, by implementing a sustainable access strategy, the industry’s reputation may continue to benefit from positive momentum.
Sources:
[i] https://theharrispoll.com/what-a-company-does-more-important/
[ii] https://www.reuters.com/article/us-health-coronavirus-investors-pharmace-idUSKBN21Z1WZ?mod=article_inline
[iii] https://milkeninstitute.org/covid-19-tracker





